![]() Objective
Objective
The objective of this lab is to use an acid with a known normality to determine the normality of a base. To accomplish this a system of titration will be used. After the normality of the base is determined, the base will be used to in turn determine the normality of another acid.
![]() Apparatus
Apparatus
A) Equipment Used
1) Flask x3
2) Sulfuric acid of a known concentration
3) Sodium Hydroxide of an unknown concentration
4) Phenolphthalein
5) Burette
6) Sodium Hydroxide of an unknown concentration
7) Stand
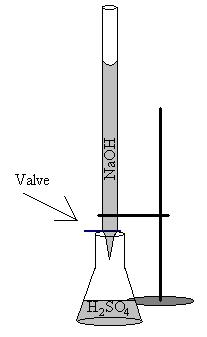
B) Diagram
![]() Method
Method
A) Part 1: Determining the normality of the base
1) Obtain and clean three flasks
2) Measure 25 ml of sulfuric acid and pour into each flask
3) Place 1 to 3 drops of phenolphthalein into each flask
4) Fill the burette approximately half full of the sodium hydroxide
5) Ensure that the tip of the burette has no bubbles by allowing a small portion of the sodium hydroxide to flow through
6) Configure using the first flask the diagram pictures above
7) Record the level of the burette
8) Begin the flow of sodium hydroxide into the flask containing the acid
9) Continue the flow until the solution with the acid changes color to pink
10) Record the final reading on the burette
11) Repeat steps 7 through 10 using flasks 2 and three
B) Part 2: Determining the normality of the acid
1) Clean and dry the three flasks
2) Fill each flask with 25 ml of the unknown acid
3) Place 1 to 3 drops of phenolphthalein into each flask
4) Record the amount of sodium hydroxide present in the burette
5) Allow the sodium hydroxide to flow into the flask until the acid changes from clear to pink
6) Record the level of sodium hydroxide in the burette
7) Repeat steps 6 and 7 for each flask
![]() Data
Data
1) Table 1: Burette readings for part A:
|
Trial: |
1 |
2 |
3 |
|---|---|---|---|
|
Initial |
25.8ml |
28.8ml |
31.0ml |
|
Final |
28.8ml |
31.0ml |
33.5ml |
2) Table 2: Burette reading for part B:
|
Trial: |
1 |
2 |
3 |
|---|---|---|---|
|
Initial |
33.5ml |
36.5ml |
39.6ml |
|
Final |
36.5ml |
39.6ml |
42.6ml |
3) Normality of acid used: .10000N
4) Initial volume of burette for part A: 25.8ml
5) Initial volume of burette for part B: 33.5ml
![]() Results
Results
6) Table 3: Acid used in reaction for part A:
|
Trial |
1 |
2 |
3 |
|---|---|---|---|
|
Volume |
25ml |
25ml |
25ml |
7) Table 4: Equivalent acid used for part A in moles:
|
Trial |
1 |
2 |
3 |
|---|---|---|---|
|
Amount |
.00125mole |
.00125mole |
.00125mole |
8) Table 5: Volume of base used in part A:
|
Trial |
1 |
2 |
3 |
|---|---|---|---|
|
Volume |
3.0ml |
2.2ml |
2.5ml |
9) Table 6: Normality of base used in part A:
|
Trial |
1 |
2 |
3 |
|---|---|---|---|
|
Normality |
.833N |
1.14N |
1.00N |
10) Average Normality: .991N
11) Table 7: Volume of base used in Part B:
|
Trial |
1 |
2 |
3 |
|---|---|---|---|
|
Volume |
3.0ml |
3.1ml |
3.0ml |
12) Table 8: Molar equivalent of base used:
|
Trial |
1 |
2 |
3 |
|---|---|---|---|
|
Moles |
.00297mole |
.00307mole |
.00297mole |
13) Table 9: Volume of unknown acid used in part B:
|
Trial |
1 |
2 |
3 |
|---|---|---|---|
|
Volume |
25ml |
25ml |
25ml |
14) Table 10: Normality of acid used in part B:
|
Trial |
1 |
2 |
3 |
|---|---|---|---|
|
Normality |
.0594N |
.0614N |
.0594N |
15) Average Normality of base: .0601N
![]() Conclusions
Conclusions
From this lab it is concluded that the base used had a normality of approximately .991N, and the acid used had a normality of approximately .0601N.
![]() Attachments
Attachments