![]() Objective
Objective
The objective of this lab is to determine the molecular weight of an unknown substance. This will be done by observing the effect that a known amount of it has on the freezing point of naphthalene.
![]() Apparatus
Apparatus
A) Equipment Used:
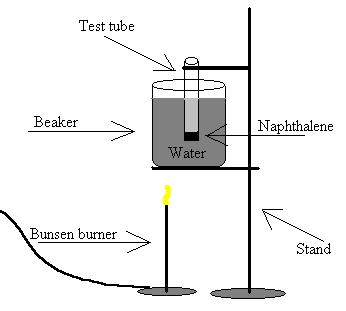
1) Beaker
2) Test tube
3) Stand
4) Bunsen burner
5) Weighing paper x2
6) Naphthalene
B) Diagram
![]() Method
Method
1) Assemble the diagram
2) Mass a piece of weighing paper
3) Use the paper to measure out 10g of naphthalene
4) Pour the naphthalene into the test tube
5) Bring the water in the beaker to boil
6) Lower the test tube containing the naphthalene into the boiling water
7) Keep the test tube submerged in the boiling water until the naphthalene melts completely.
8) Immediately begin taking temperature readings once the naphthalene has been removed
9) Take one temperature reading every minute for 14 minutes
10) Mass an additional piece of weighing paper
11) Mass 1g of the unknown solute using the weighing paper and add it to the naphthalene
12) Place the test tube back into the boiling water and allow the mixture to melt again
13) Remove the test tube from the boiling water and begin taking temperature readings
14) Take a temperature readings for 14 minutes in one minute intervals
![]() Data
Data
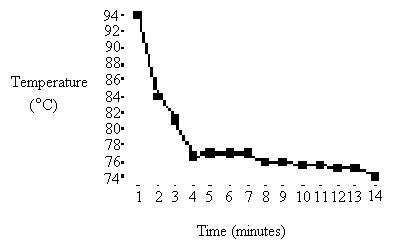
1: Table 1: Temperature table from first heating:
|
Time: |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Temp: |
94 |
81 |
77 |
79 |
78 |
79 |
79 |
79 |
79 |
79 |
78.5 |
78 |
78 |
77 |
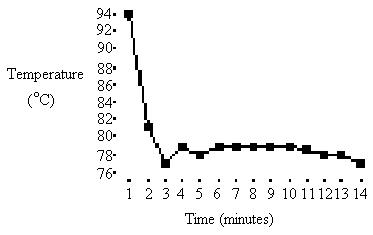
2: Table 2: Temperature table from second heating:
|
Time: |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Temp: |
94 |
84 |
81 |
76.5 |
77 |
77 |
77 |
76 |
76 |
75.5 |
75.5 |
75 |
75 |
74 |
3) Mass of naphthalene: 10.000g
4) Mass of solvent: 1.000g
![]() Results
Results
1) Freezing point of pure naphthalene: 79OC
2) Freezing point of solution: 77OC
3) Freezing point lowering: 2OC
4) Moles of solute per Kg of solution: .29 mole / Kg
5) Mass of naphthalene in solution: 10.000g
6) Mass of solvent in solution: 1.000g
7) Mass of solute per Kg of solution: 100g / Kg
8) Molecular mass of solute: 345 g / mole
9) Number of atoms in sulfur ring: 11 atoms
10) Graph of Temperature vs Time for first cooling:
11) Graph of Temperature vs Time for second cooling: