![]() Objective
Objective
CH - 120
Fall 2000
Molecular Weight of a Condensable Vapor
Experiment #6
Submitted by:
Andrew J. Buettner
Partners:
Adam Stevens
Joe Breczinski
Report
Due:
![]() Objective
Objective
The objective of this lab is to determine the molecular mass of a substance.
![]() Apparatus
Apparatus
A) Equipment used
1) Aluminum foil
2) 125mL flask
3) Beaker
4) Stand
5) Bunsen burner
6) Methanol
B) Diagram
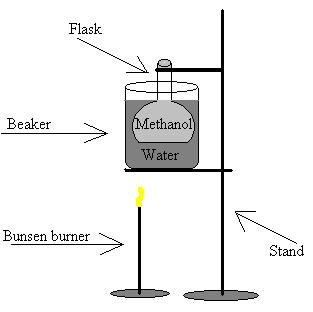
![]() Method
Method
1) Use some aluminum foil and create a cap for the flask
2) Poke a small hole in the top of the cap
3) Mass the flask and cap
4) Pour 3ml of methanol
5) Place the lid back on the flask
6) Fill the beaker with water and bring it to boiling
7) Use the stand to suspend the flask in the boiling water
8) Allow the methanol to evaporate
9) Record the temperature of the water
10) Allow the liquid to condense in the flask
11) Mass the flask, cap, and condensed liquid
12) Fill the flask with water and record the volume
![]() Data
Data
1) Mass of flask and cap: 108.715g
2) Mass of flask, liquid, and cap after heating: 108.952g
3) Temperature of boiling water: 100.5OC
4) Barometric pressure: 786torr
5) Volume of flask: 276.1mL
![]() Results
Results
1) Mass of condensed liquid: .237g
2) Volume of vapor at STP: 208.7mL
3) Number of moles of vapor: .009320mole
4) Molecular mass of vapor: 25.4g/mole
![]() Answers to Lab Questions
Answers to Lab Questions
1) Q: What does the flask contain before adding the liquid, when the liquid has vaporized, and during the final massing.
A: Before adding the liquid, there was only air from the room; after the liquid had vaporized all of the air had been completely displaced, leaving only methanol; At the end, there was methanol vapor, methanol liquid, and air.
2) Q: Why is the temperature of the boiling water used in the calculations instead of the room temperature?
A: The temperature of the water is the temperature of the gas when it has completely filled the container, after it has cooled, it no longer completely fills the container.
3) Q: When vaporized, the liquid does not behave like an ideal gas, what direction would the deviation be expected, and what impact would it have on the molecular weight?
A: The gas would probably be more expansive than the ideal gas law causing the calculated molecular weight to be lower than the actual molecular weight.
4) Q: What are the restrictions would the liquid used for this lab be?
A: First, it has to be able to vaporize below the boiling point of water, second, in it's vapor form, it has to be heavier than air as to not escape.
![]() Conclusions
Conclusions
From this lab it is determined that the molecular mass of methanol is 25.4g/mole. This value contains 20.6% error from the actual scientific value of 32.0g/mole for methanol. This large error is the result of water vapor introducing it's self into the system as well as air it's self.
![]() Attachments
Attachments
1) Original lab data
2) Calculations